
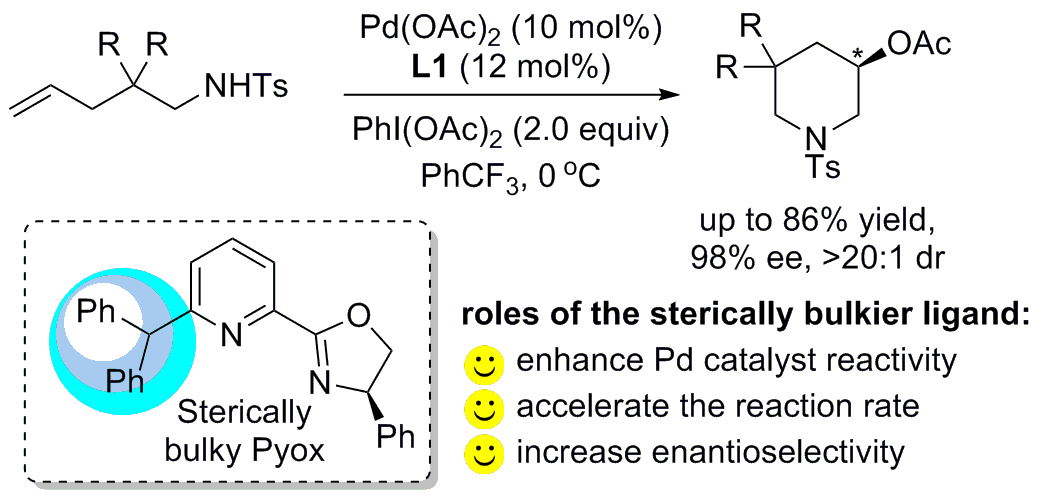
Asymmetric 1,n-Remote Aminoacetoxylation of Unactivated Internal Alkenes Enabled by Palladium Catalysis
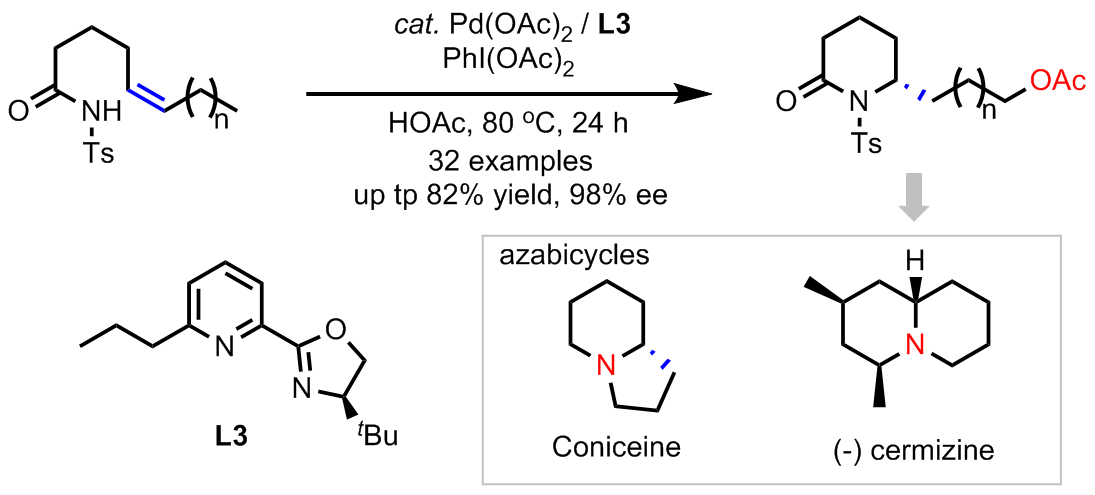
A palladium-catalyzed asymmetric 1,n-remote aminoacetoxylation of cis-alkenes has been developed using PhI(OAc)2 as an oxidant, providing the acetoxylated lactams with excellent enantioselectivities under mild reaction conditions. The sterically hindered pyridine-oxazoline (Pyox) L3 with a tert-butyl group in oxazoline ring and propyl group in C6 position of pyridinyl is vital for the reaction, where the former is good for asymmetric aminopalladation step and the latter for the chain walking process. The enantioenriched lactam products were proven to be good building blocks for the synthesis of azabicycles.This result is published in Angew. Chem. Congratulations Xintuo.

Site-Selective sp2 C–H Cyanation of Allenes via Copper-Catalyzed Radical Relay

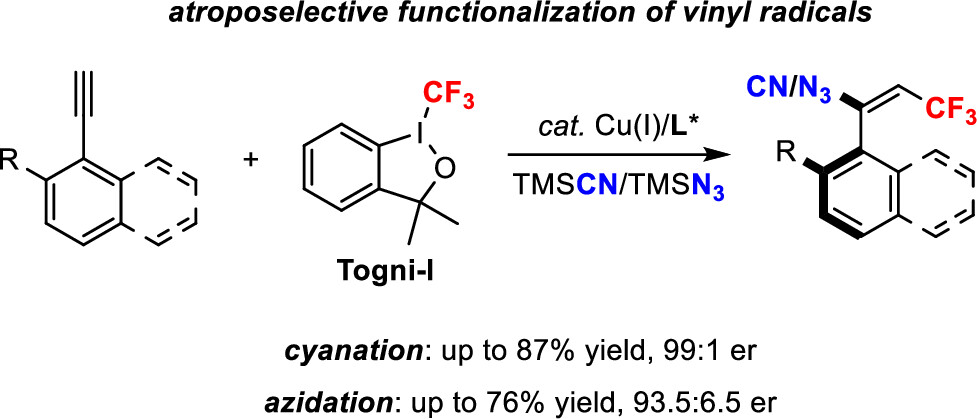
Copper-Catalyzed Asymmetric Functionalization of Vinyl Radicals for the Access to Vinylarene Atropisomers

Congratulations

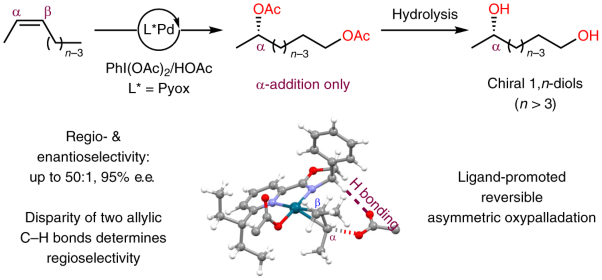
Regio- and enantioselective remote dioxygenation of internal alkenes

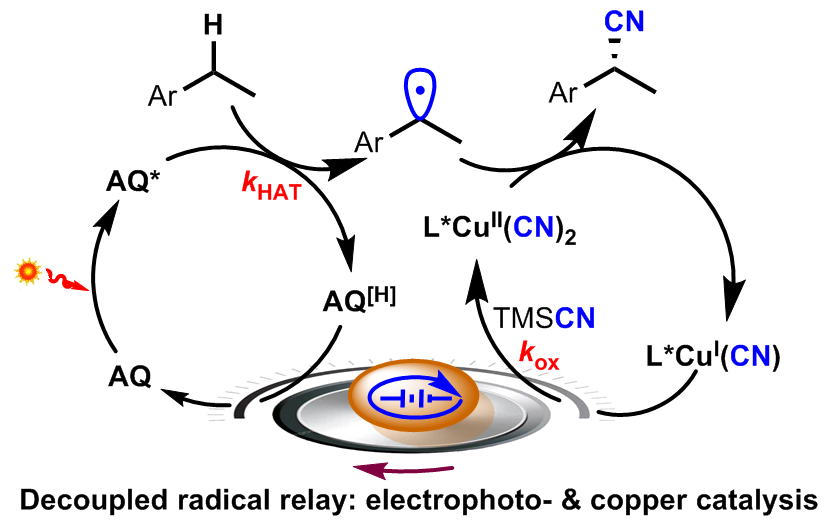
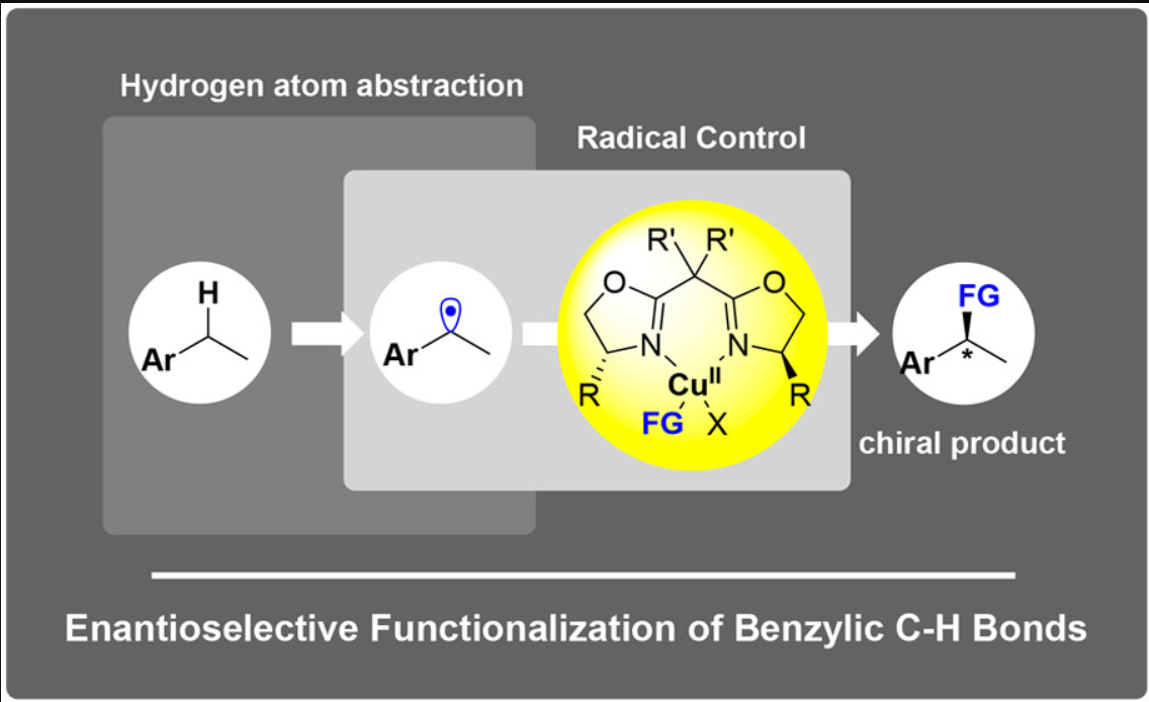
Electrophotocatalytic Decoupled Radical Relay Enables Highly Efficient and Enantioselective Benzylic C–H Functionalization

Palladium-Catalyzed Remote Hydrooxygenation of Internal Alkenes: An Efficient Access to Primary Alcohols

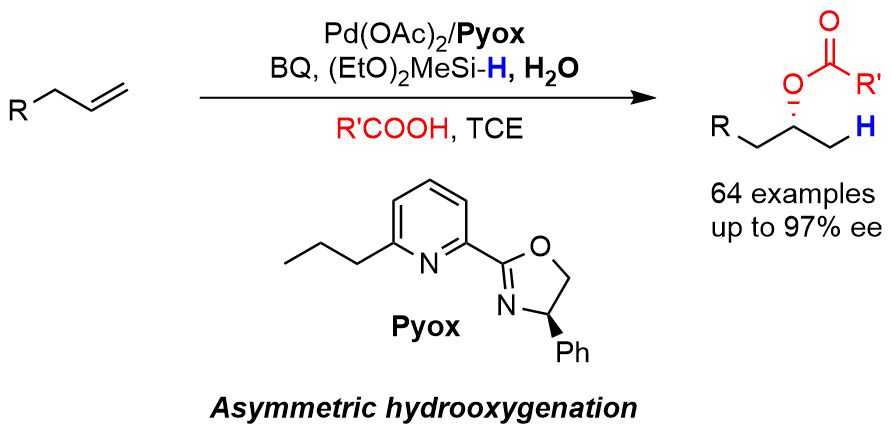
Palladium(II)-Catalyzed Enantioselective Hydrooxygenation of Unactivated Terminal Alkenes
Copper-catalyzed radical relay in C(sp3)–H functionalization
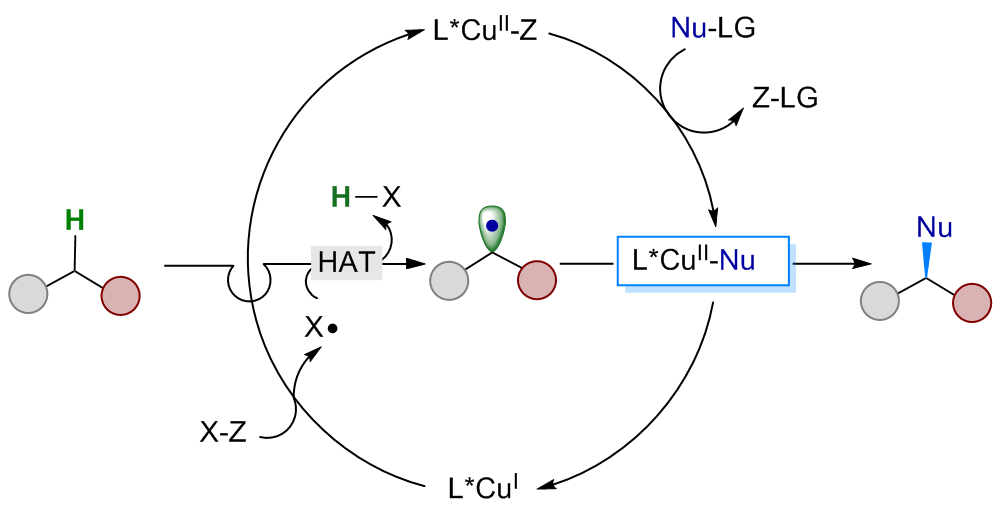

Copper-catalysed asymmetric radical cyanation
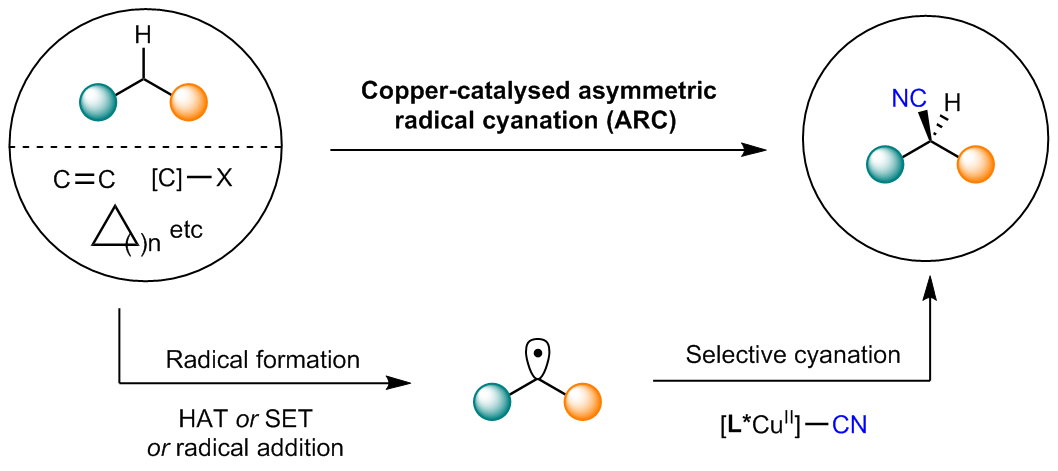

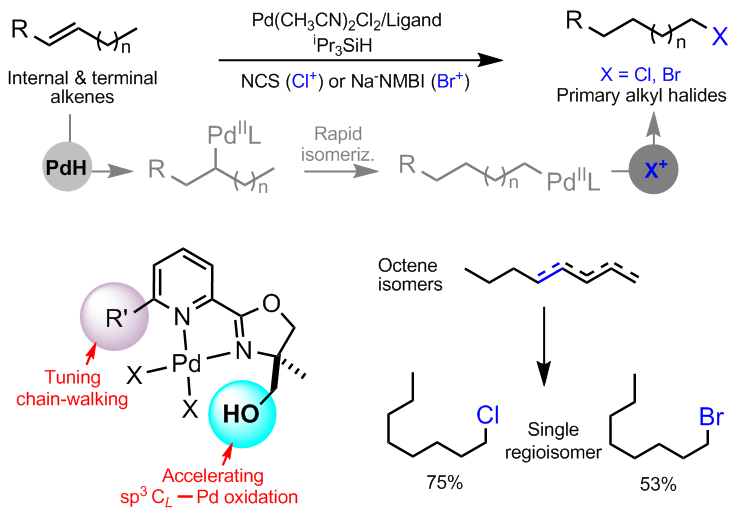
Catalytic remote hydrohalogenation of internal alkenes


Enantioselective Copper-Catalyzed Radical Cyanation of Propargylic C–H Bonds: Easy Access to Chiral Allenyl Nitriles


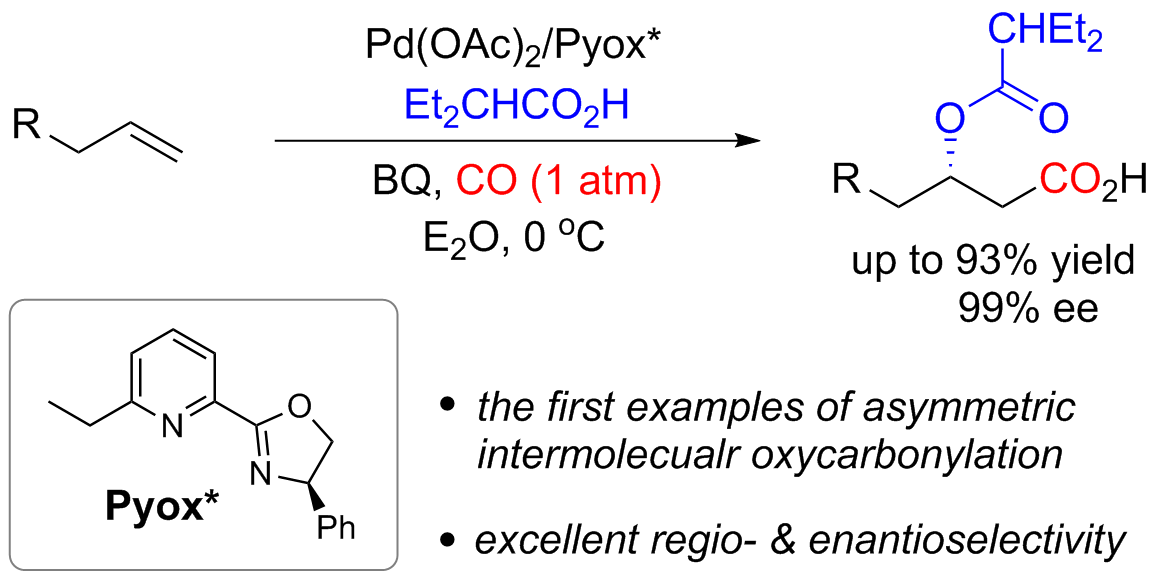
Asymmetric Palladium-Catalyzed Oxycarbonylation of Terminal Alkenes: Efficient Access to β-Hydroxy Alkylcarboxylic Acids

Palladium-catalysed enantioselective diacetoxylation of terminal alkenes
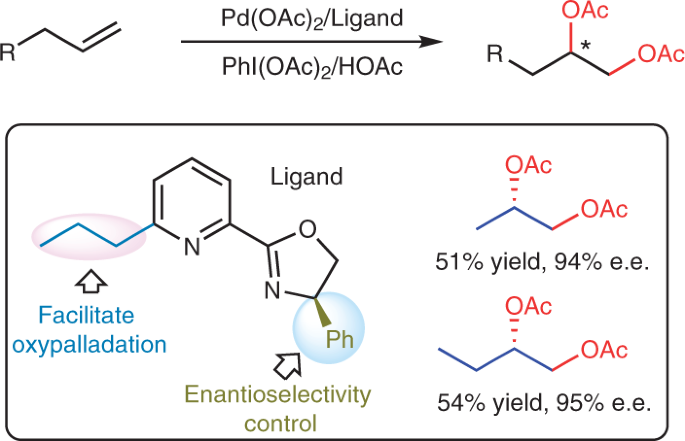

Anionic Bisoxazoline Ligands Enable Copper-Catalyzed Asymmetric Radical Azidation of Acrylamides
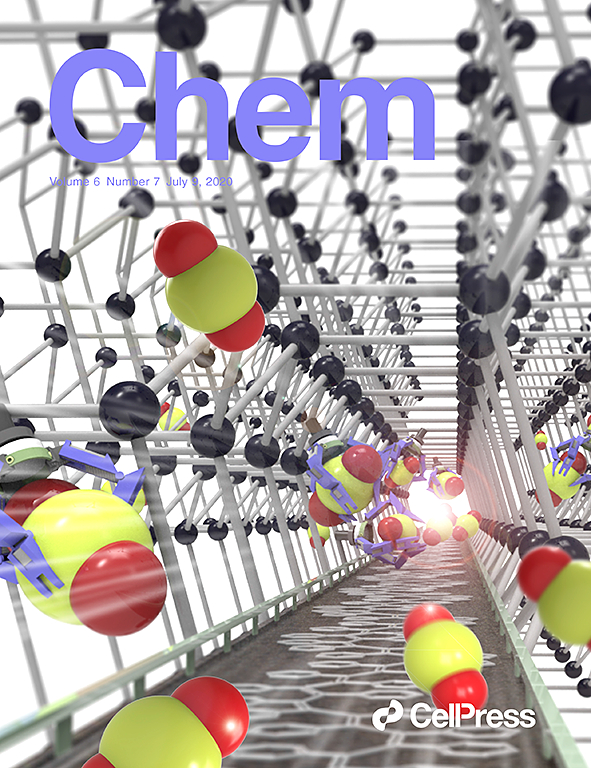
Enantioselective Copper-Catalyzed Trifluoromethylation of Benzylic Radicals via Ring Opening of Cyclopropanols

Asymmetric Coupling of Carbon-Centered Radical Adjacent to Nitrogen: Copper-Catalyzed Cyanation and Etherification of Enamides


Enantioselective Copper-Catalyzed Alkynylation of Benzylic C-H Bonds via Radical Relay


Palladium(II)-Catalyzed Enantioselective Azidation of Unactivated Alkenes
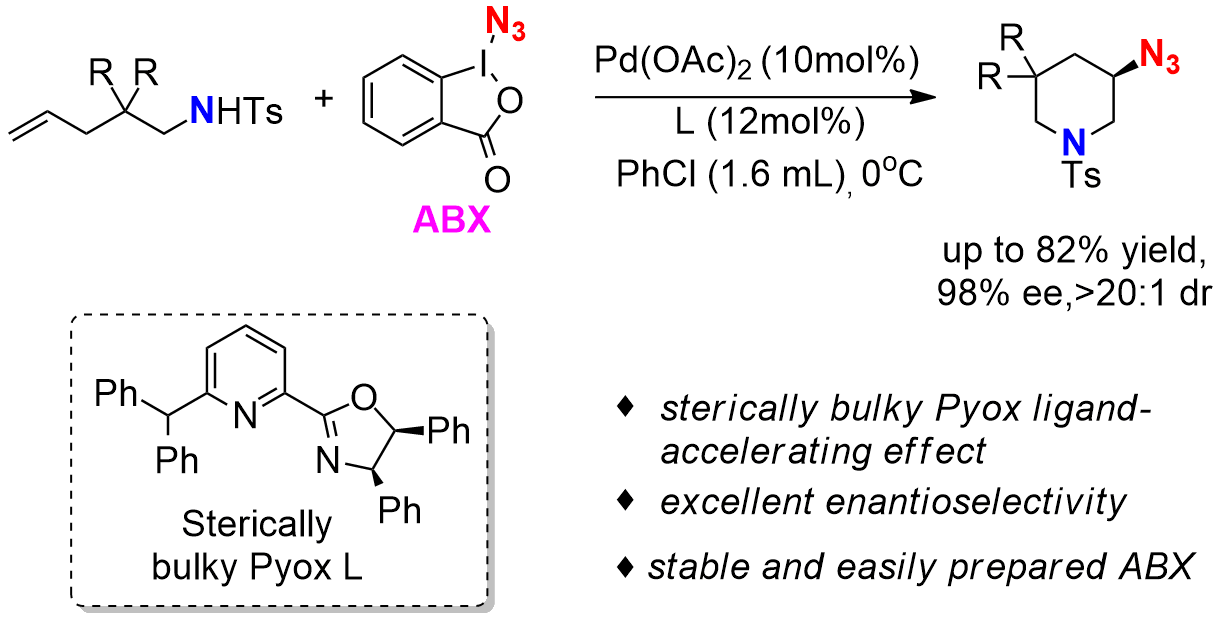

Enantioselective Pd(II)-Catalyzed Oxidative Aminofluorination of Unactivated Alkenes Using Et4NF·3HF as a Fluoride Source

Congratulations

Welcome

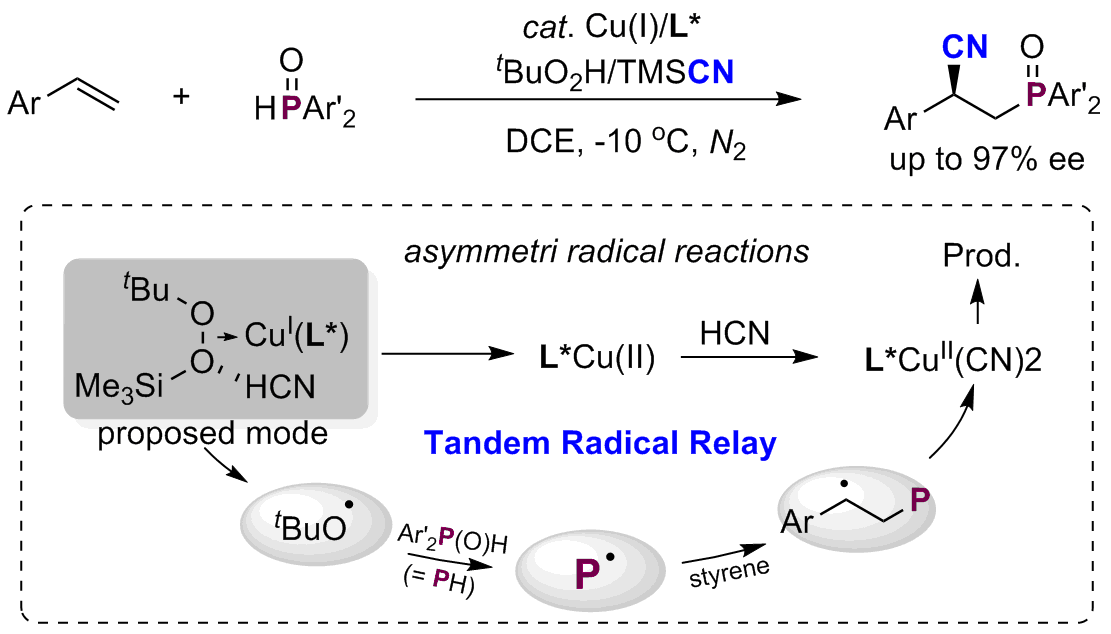
Proton-Coupled Electron Transfer Enables Tandem Radical Relay for Asymmetric Copper-Catalyzed Phosphinoylcyanation of Styrenes

Enantioselective Arylation of Benzylic C-H bonds via CopperCatalyzed Radical Relay

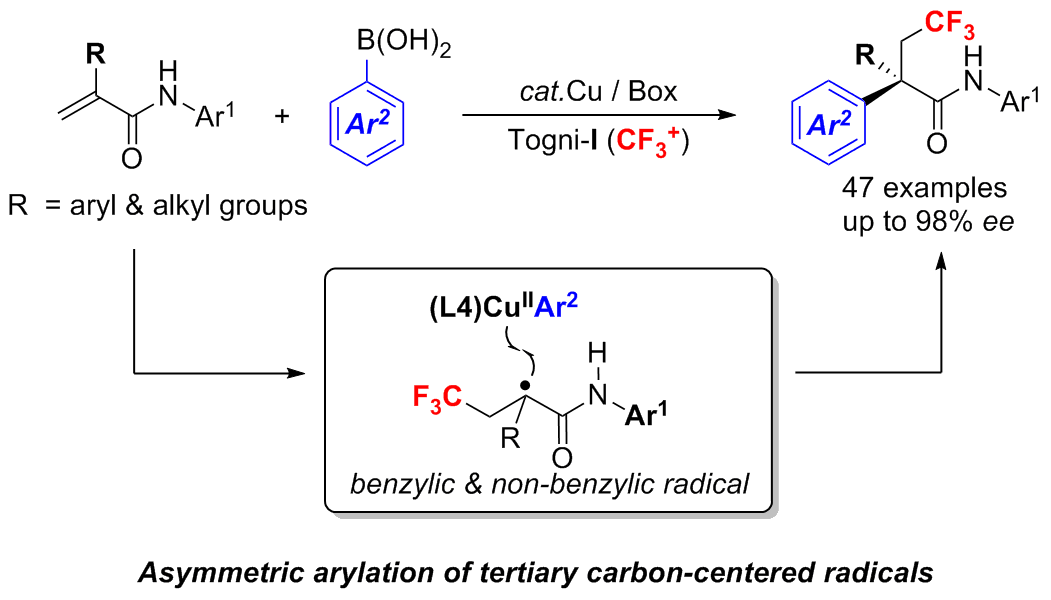
Enantioselective Construction of Quaternary All-Carbon Centers via Copper-Catalyzed Arylation of Tertiary Carbon-Centered Radicals

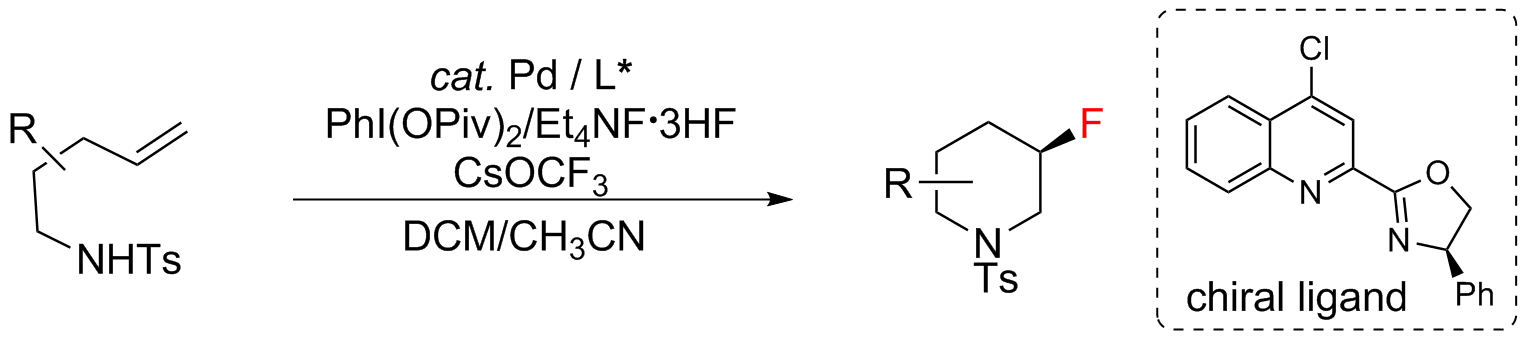
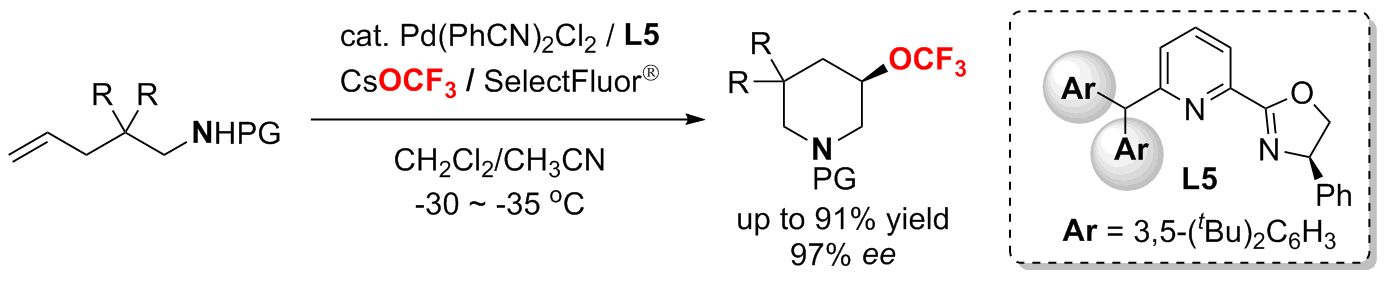
Palladium(II)-Catalyzed Enantioselective Aminotrifluoromethoxylation of Unactivated Alkenes using CsOCF3 as a Trifluoromethoxide Source

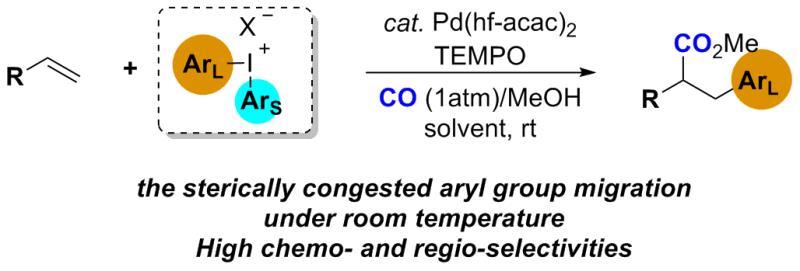
Pd-Catalyzed Intermolecular Arylcarbonylation of Unactivated Alkenes: Incorporation of Bulky Aryl at Room Temperature
Enantioselective Trifluoromethylalkynylation of Alkenes via Copper-Catalyzed Radical Relay
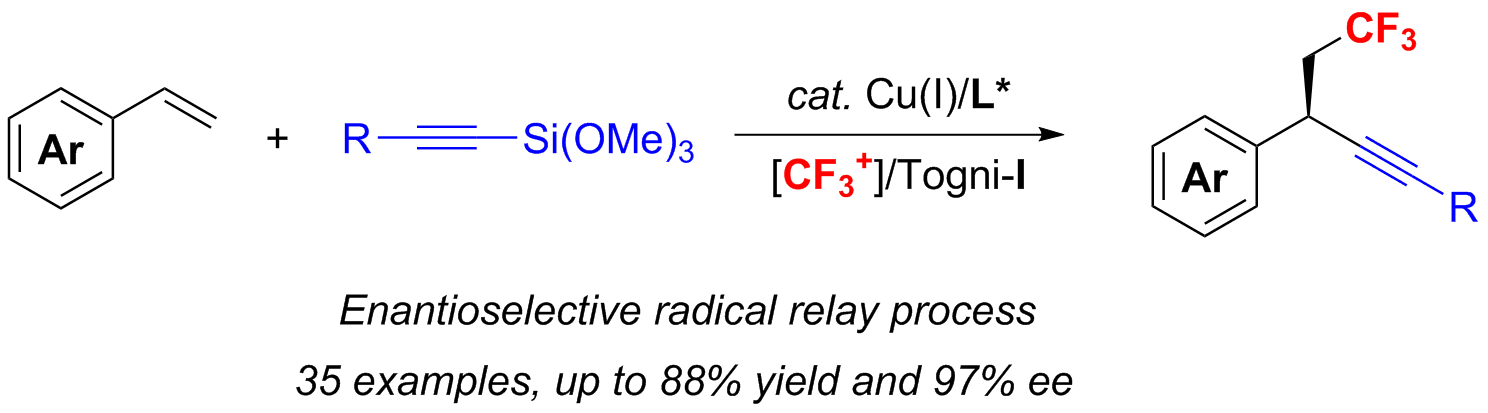

Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations

Welcome

Enantioselective Pd(II)-Catalyzed Intramolecular Oxidative 6-endo Aminoacetoxylation of Unactivated Alkenes

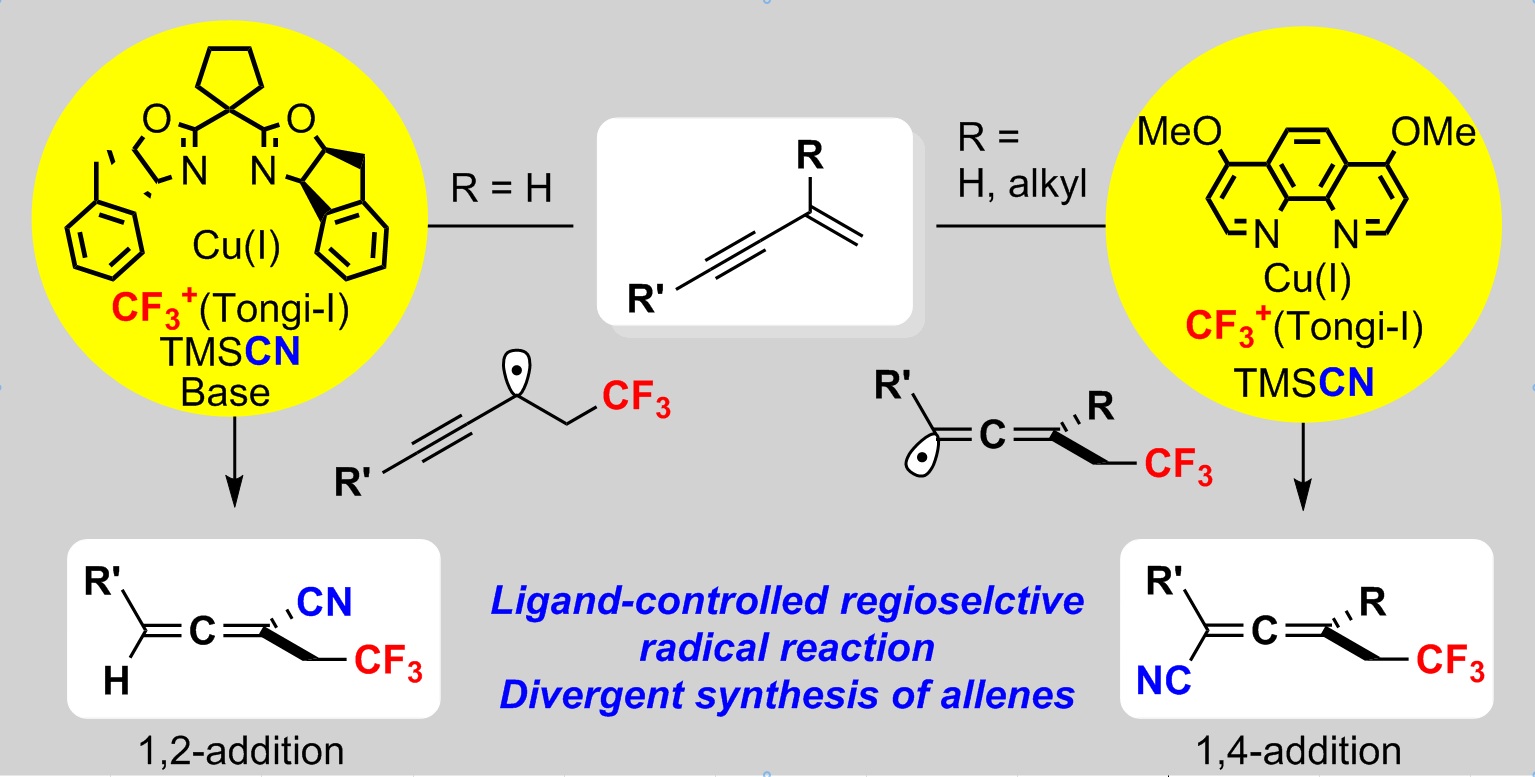
Divergent Synthesis of CF3-Substituted Allenyl Nitriles by Ligand-Controlled Radical 1,2- and 1,4-Addition of 1,3-Enynes

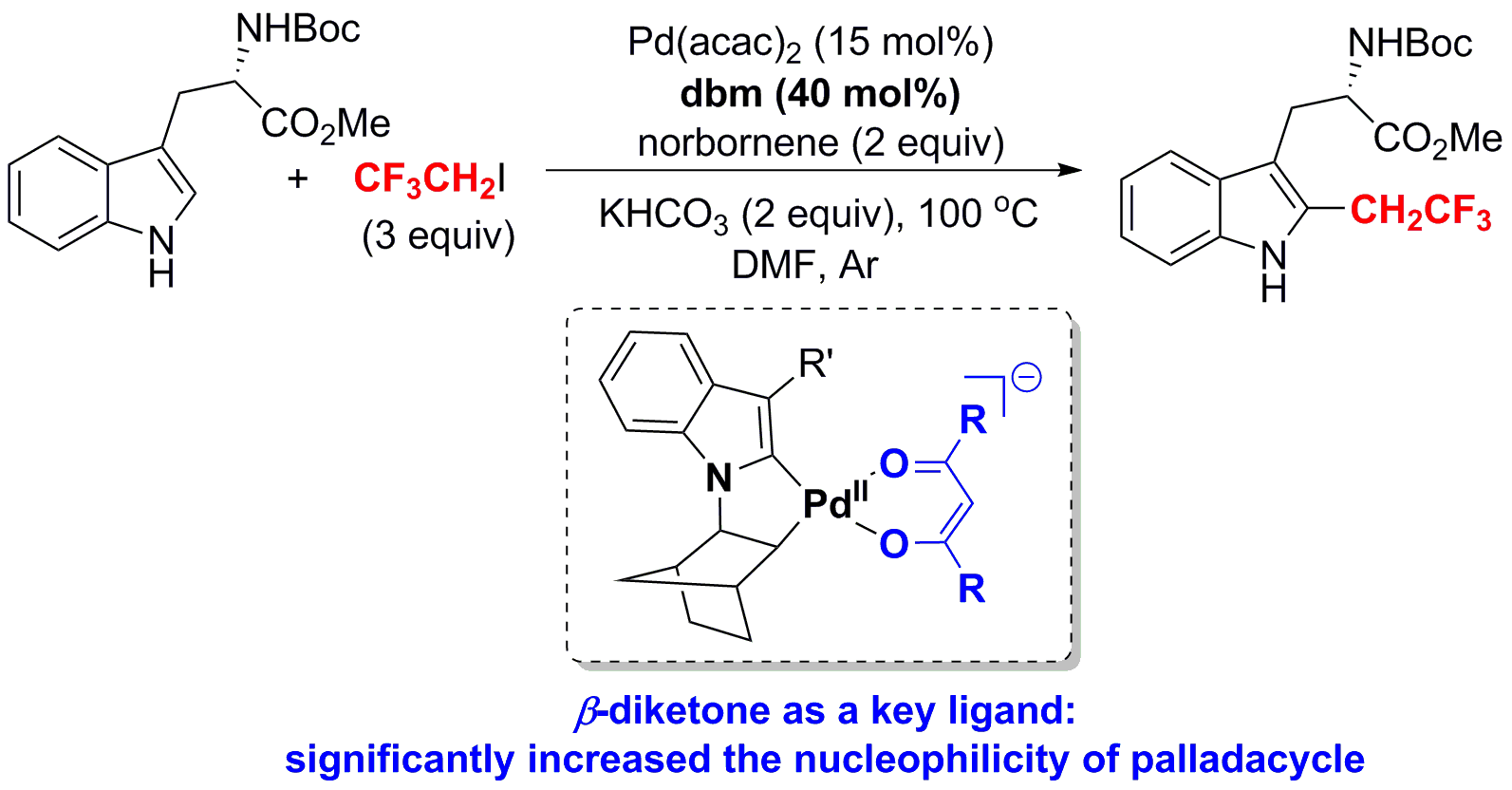
Regioselective Palladium-Catalyzed C-H Bond Trifluoroethylation of Indoles: Exploration and Mechanistic Insight

Welcome

Welcome

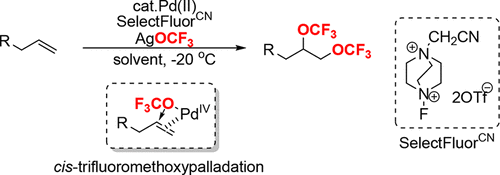
Palladium-Catalyzed Intermolecular Ditrifluoromethoxylation of Unactivated Alkenes: CF3O?Palladation Initiated by Pd(IV)

Awards Notification
Enantioselective Decarboxylative Cyanation Employing Cooperative Photoredox Catalysis and Copper Catalysis


Palladium-Catalyzed Intermolecular Azidocarbonylation of Alkenes via a Cooperative Strategy
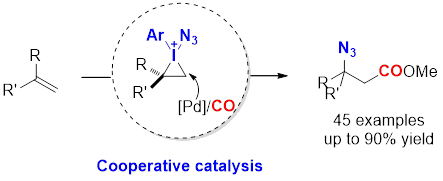

Palladium-Catalyzed Intermolecular Oxidative Fluorocarbonylation of Unactivated Alkenes: Efficient Access of β-Fluorocarboxylic esters

Awards Notification

Congratulations

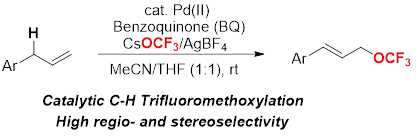
Catalytic Oxidative Trifluoromethoxylation of Allylic C-H Bonds using a Palladium Catalyst

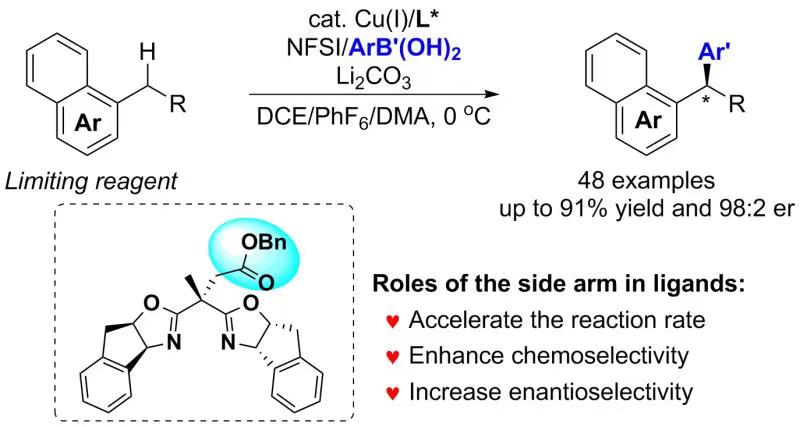
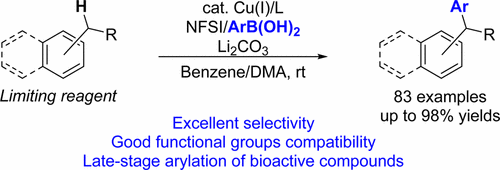
Copper-Catalyzed Arylation of Benzylic C–H bonds with Alkylarenes as the Limiting Reagents

Asymmetric Copper-Catalyzed Intermolecular Aminoarylation of Styrenes: Efficient Access to Optical 2,2-diarylethylamines
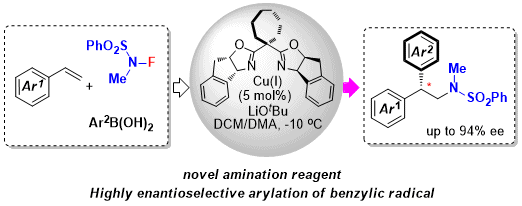

Welcome
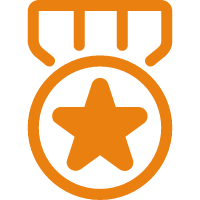
Awards Notification

Enantioselective Palladium(II)-Catalyzed Intramolecular Aminoarylation of Alkenes by Dual N-H and Aryl C-H Bond Cleavage
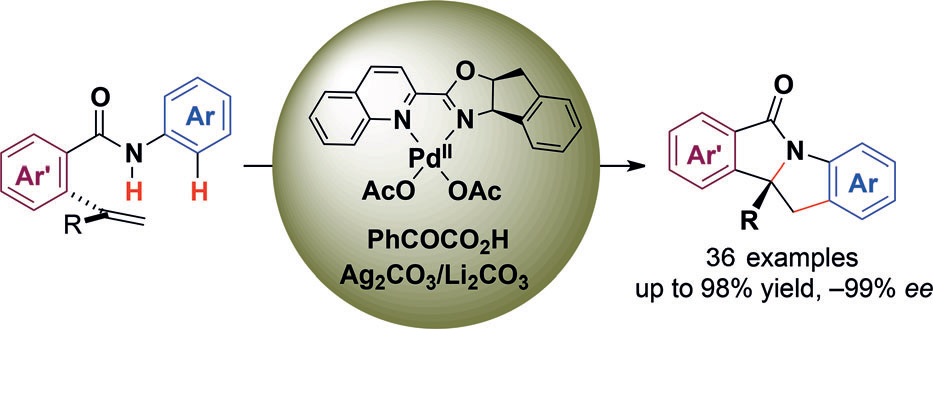
Asymmetric Cu-Catalyzed Intermolecular Trifluoromethylarylation of Styrenes: Enantioselective Arylation of Benzylic Radicals
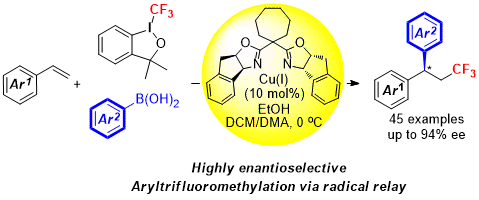

Enantioselective Copper-Catalyzed Intermolecular Amino- and Azido-cyanation of Alkenes via radical relay process

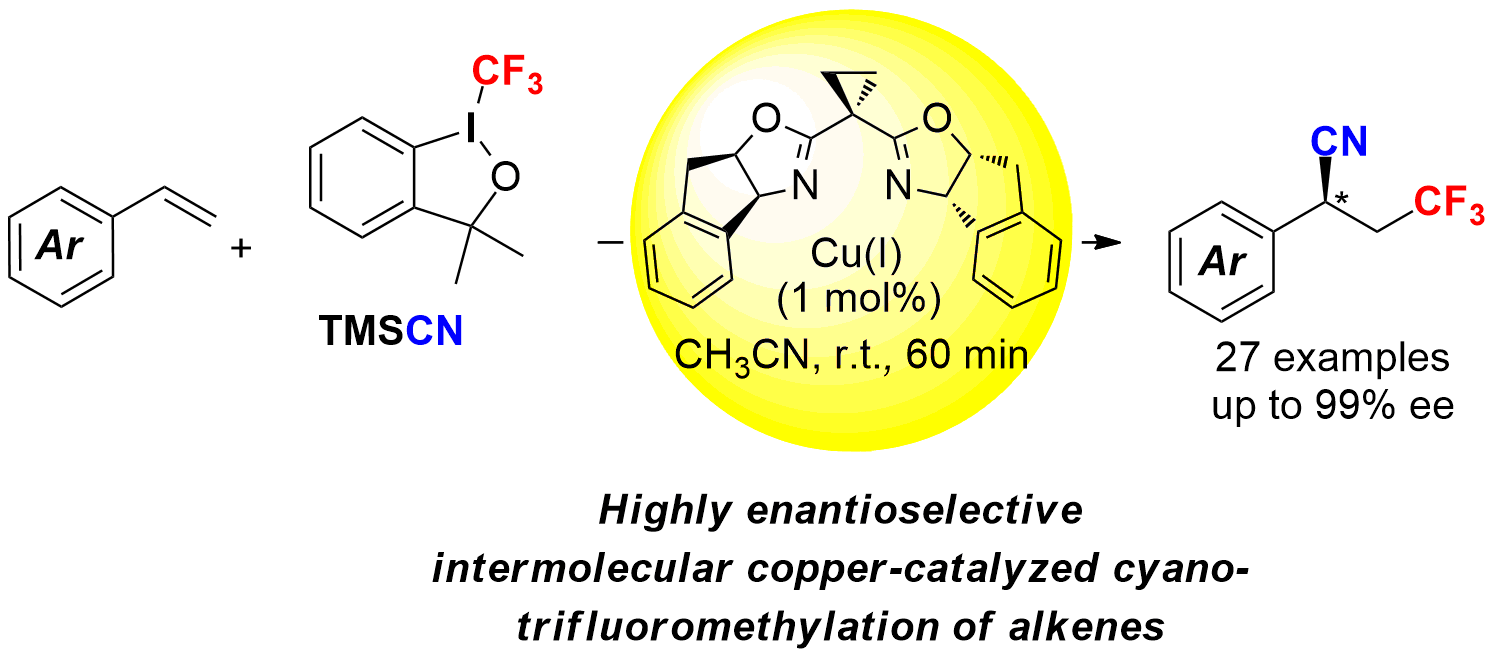
Enantioselective Copper-Catalyzed Intermolecular Cyanotrifluoromethylation of Alkenes via Radical Process

Author Profile of Guosheng Liu in Angewandte Chemie

Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center
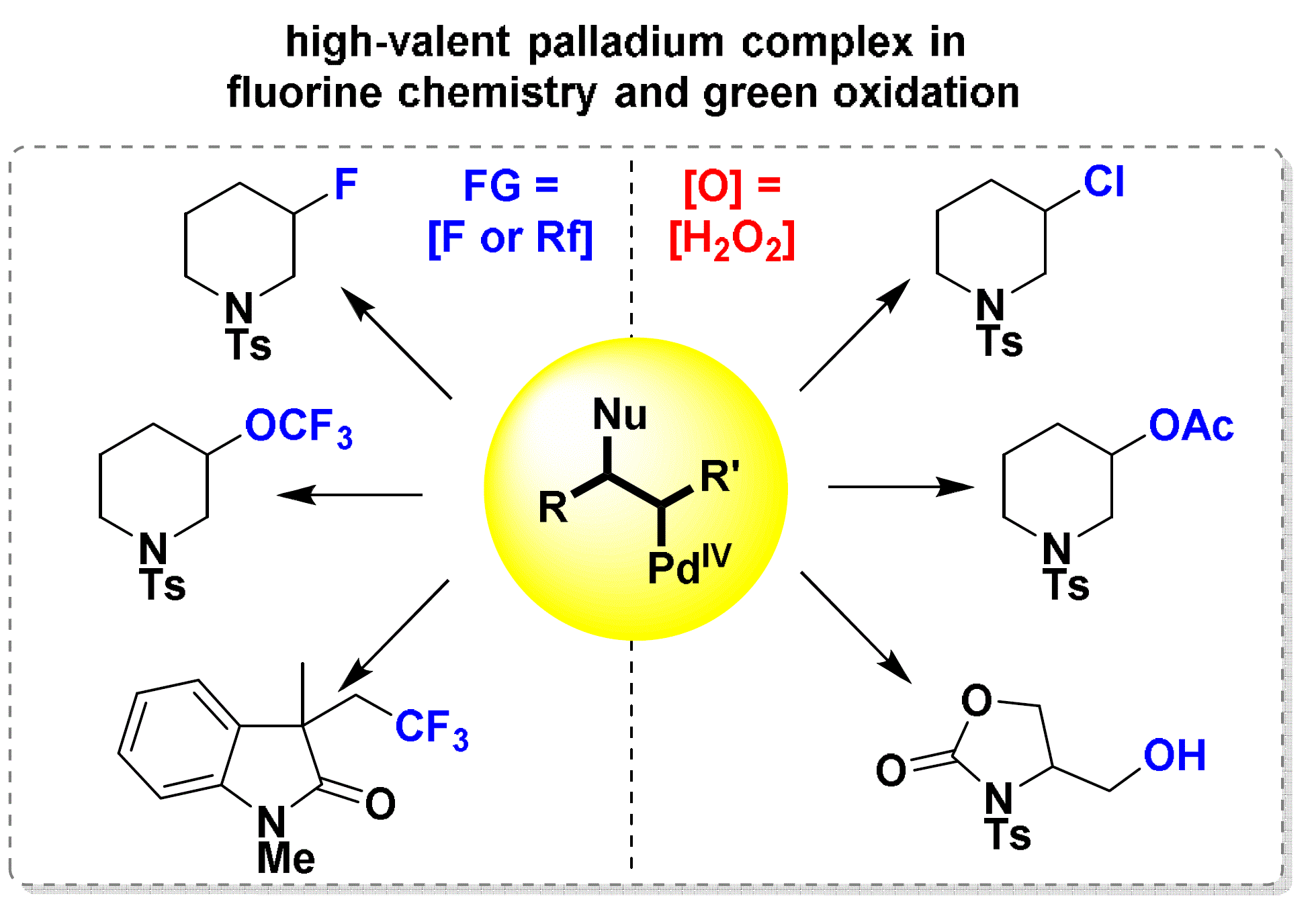

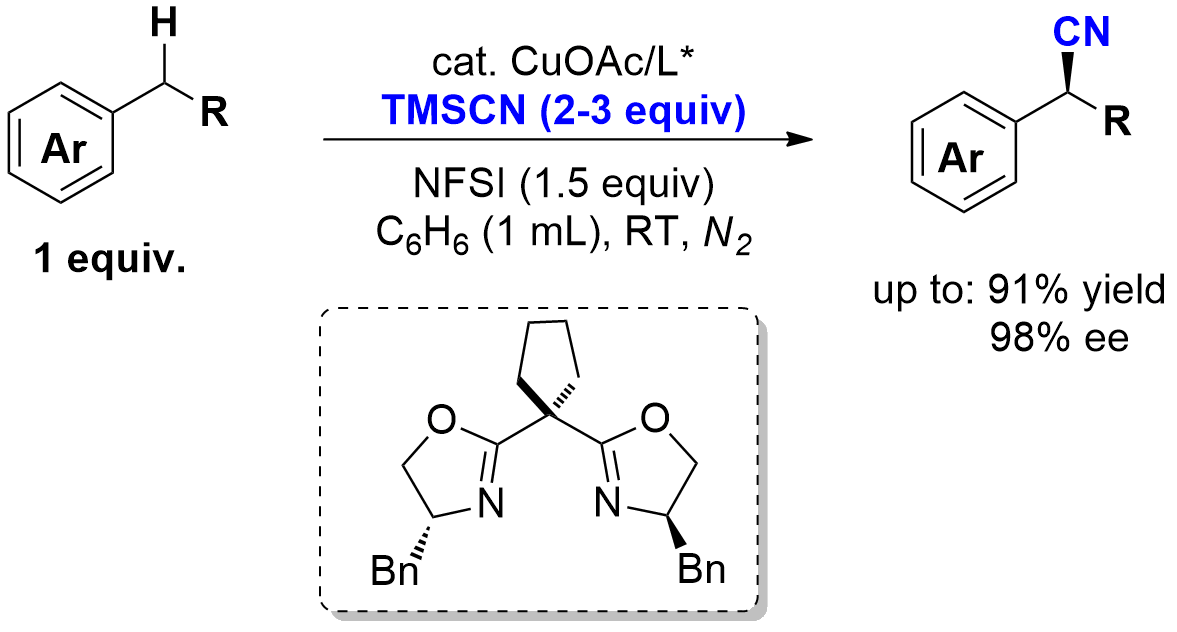
Congratulations Wen and Fei.

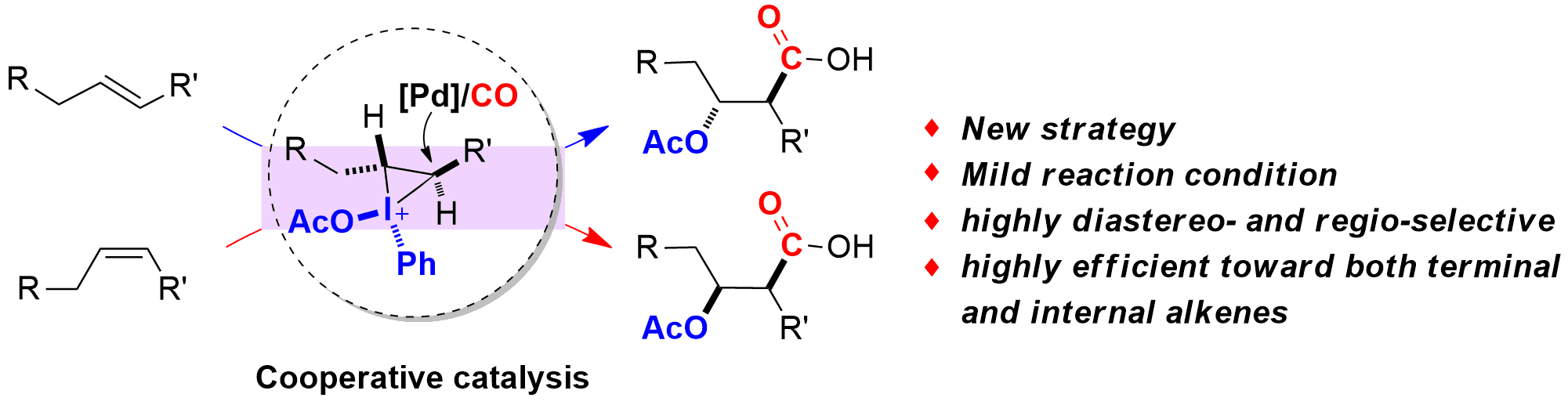
Cooperative Strategy for the Highly Selective Intermolecular Oxycarbonylation Reaction of Alkenes using palladium catalyst

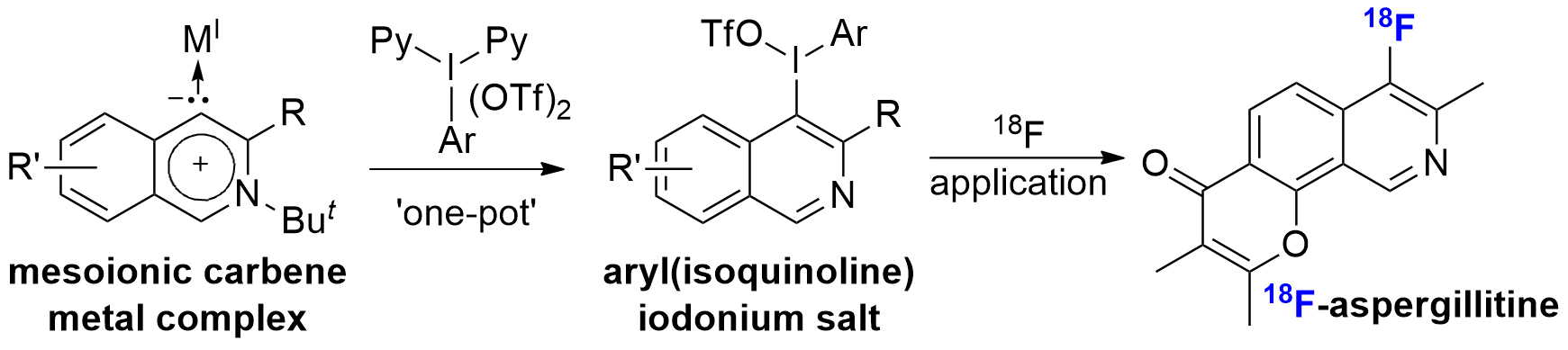
Efficient Pathway for the Preparation of Aryl(isoquinoline)iodonium(III) Salts and Synthesis of Radiofluorinated Isoquinolines

 Postdoctoral positions are available immediately in the Prof. Liu’s lab. Interested candidates with strong experimental experience on organic chemisty, especially on radical chemistry and transition metal catalysis, please submit a cover letter, Curriculum Vitae (including 2x references) and a short research summary to Prof. Liu, gliu@mail.sioc.ac.cn. Please put "Applications - Liu Group - PostDoc" in the subject line.
Postdoctoral positions are available immediately in the Prof. Liu’s lab. Interested candidates with strong experimental experience on organic chemisty, especially on radical chemistry and transition metal catalysis, please submit a cover letter, Curriculum Vitae (including 2x references) and a short research summary to Prof. Liu, gliu@mail.sioc.ac.cn. Please put "Applications - Liu Group - PostDoc" in the subject line.